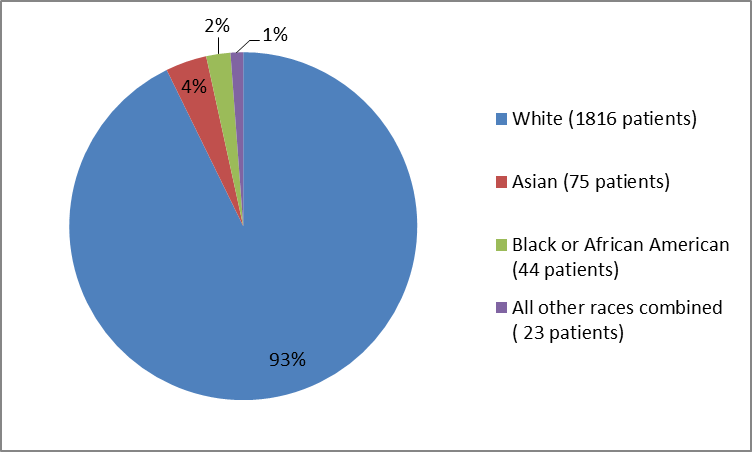
Adverse reactions that occurred at rates 1 in the ixekizumab group and more frequently than in the placebo group during the 12 week induction period included rhinitis oral candidiasis urticaria influenza conjunctivitis inflammatory bowel disease and angioedema.
Taltz lilly side effects.
Taltz must not be given to patients who have potentially serious infections such as tuberculosis.
Injection site reactions redness pain upper respiratory tract infections runny or stuffy nose rhinovirus infections.
Taltz is also used in adults to treat axial spondyloarthritis.
Common symptoms of plaque psoriasis include.
Body aches or pain.
Side effects requiring immediate medical attention.
Low levels of a type of white blood cell called neutrophils.
Discharge or excessive tearing.
Common side effects of taltz include.
Weight gain is not a known side effect of taltz ixekizumab a humanized interleukin 17a antagonist used to treat adults and children with moderate to severe plaque psoriasis as well as adults with active psoriatic arthritis ankylosing spondylitis and non radiographic axial spondyloarthritis with objective signs of inflammation.
Taltz is used in adults to treat active psoriatic arthritis or active ankylosing spondylitis.
A following 160 mg starting dose of ixekizumab at week 0.
The most common side effects with taltz which may affect more than 1 in 10 people are pain and redness at the injection site and nose throat or chest infections.
Burning dry or itching eyes.
Inflammation of the large intestine.
Patches commonly appear on the knees elbows lower back scalp and genitalia.
Common side effects of taltz include.
Patches may also appear on other areas of the body.
Raised red silver or flaky scaly patches on your skin.
Injection site reactions upper respiratory tract infections nausea tinea infections such as ringworm athlete s foot and jock itch runny or stuffy nose oral thrush hives influenza and conjunctivitis.